Please contact me to discuss treatments such as anti-wrinkle injections, dermal fillers, and skin treatments.

Important Product Disclaimers
XEOMIN®
Xeomin® is a Prescription Medicine containing 50, 100 units of incobotulinum Type A, purified Botulinum toxin type A complex for injection. It is used for the treatment of frown lines on the forehead, lateral periorbital lines and horizontal forehead lines in adults. It should be administered only by trained medical professionals. Talk to your specialist about the benefits/risks of this procedure in appearance medicine. Xeomin treatment lasts about four months and further courses of treatment may be necessary. Cautions: people receiving blood thinning medicines, care at the proposed injection sites, pregnancy and lactation. Possible side effects: headache, pain, swelling or infection at injection site, local muscle weakness including drooping eye lids, lack of feeling & nausea. Treatment last for up to 4 months.
PROFHILO®
PROFHILO® containing low & high molecular weight hyaluronic acid, is a Class III medical device for the treatment of the face and body for contours redefinition and laxity remodelling where skin laxity is a problem. Profhilo® has risks and benefits. Do not use with treatments such a laser resurfacing or medium deep skin-peeling. Caution in people on blood thinning medicines. Do not inject into inflamed areas or intravenously or intramuscularly. Possible side effects: pain and swelling at injection site. Accelagen Pty. Ltd. Whanganui.
PROFHILO® STRUCTURA is a class III medical device and must be injected by a medical practitioner or a qualified nurse injector (operating under the supervision of a medical practitione
PROFHILO® STRUCTURA [4.5% – 22.5 mg (H-HA) + 22.5 mg (L-HA)/1 ml Hyaluronic acid sodium salt and 4.5% – 45 mg (H-HA) + 45 mg (L-HA)/2 ml Hyaluronic acid sodium salt], injection gel in a single use sterile prefilled syringe. It is indicated for adults of both sexes for corrective/filling action of natural or induced skin depressions.
PROFHILO® STRUCTURA has risks and benefits. Ask your healthcare professional if PROFHILO® STRUCTURA is right for you and to explain the possible side effects.
WARNINGS & PRECAUTIONS: Do not use PROFHILO® STRUCTURA in case of known hypersensitivity or allergies to the components of the product. Do not use it in pregnant or breast-feeding women, or in patients with autoimmune diseases. Do not inject intravascularly, into the muscles or tendons, or for breast enlargement.
CONTRAINDICATIONS: PROFHILO® STRUCTURA must not be used in conjunction with treatments such as laser resurfacing and medium-deep peeling. Tell your doctor if any side effects concern you.
ALWAYS FOLLOW THE INSTRUCTIONS YOU ARE GIVEN.
Treatment costs and normal practitioner fees will apply. Please contact us for any further information about the IFU (Instructions for use) of this product or for medical information or reporting any adverse effects.
SUNEKOS
Sunekos containing hyaluronic acid and amino acids is a class III medical device that modifies the structure of mature skin, restoring volume, filling wrinkles and folds in the skin and in scar sites. Sunekos has risks and benefits.
Sunekos should not be used on patients; with known hypersensitvity to any of its components; presenting with a general infection, inflammation or irritation in the area to be treated; or in patients predisposed to coagulation disorders.
A local reaction may rarely occur, caused by hyper sensitisation phenomena, with symptoms that include oedema and sensation of burning and/or itching. These reactions normally resolve within two days.
Consult your healthcare professional to see if Sunekos is right for you. For more information refer to the instructions for use. http://www.xytide.co.nz/
New Zealand sponsor: AA-Med Pty Ltd
Distributed by: Xytide Biotech NZ Pty Ltd
RESTYLANE®
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Kysse, Restylane® Refyne, Restylane® Defyne, Restylane® Contour, and Restylane® Eyelight.
Approved Uses
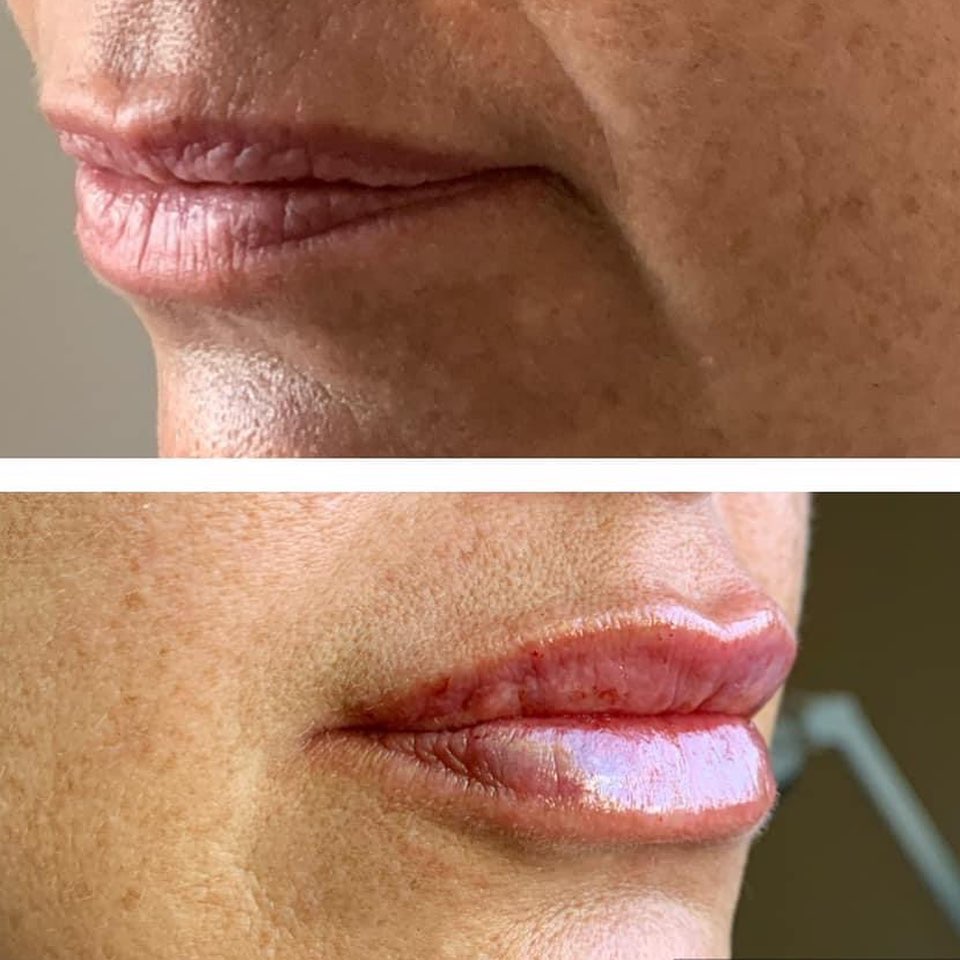
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds. Restylane® Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild to moderate chin retrusion.
Restylane® Contour is for cheek augmentation and for the correction of midface contour deficiencies.
Restylane® Eyelight is for the improvement of infraorbital hollowing.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Delayed-onset inflammation near the site of dermal filler injections is one of the known adverse events associated with dermal fillers, and cases have been reported to occur at the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. Typically, the reported inflammation was responsive to treatment or resolved on its own. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To report a side effect with any Restylane® product, please call Galderma Laboratories, L.P. at 1-855-425-8722.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
US-RES-2400145
VISCODERM® HYDROBOOSTER
To learn more about VISCODERM® HYDROBOOSTER go to www.ibsaderma.co.nz
VISCODERM® HYDROBOOSTER is a Class III medical device containing 25mg/1mL of cross-linked hyaluronic acid, it is used to restore intradermal hydration and to keep improve the structure and elasticity of the skin. VISCODERM® HYDROBOOSTER must only be administered by a medical practitioner or a qualified nurse injector (who operates under the supervision of a medical practitioner). Treatment costs and normal practitioner’s fees will apply. VISCODERM® HYDROBOOSTER has risks and benefits. If you have side effects or concerns, speak to your medical practitioner. Please consult your medical practitioner regarding its suitability for you. For further product information, please refer to your medical practitioner or the Instructions for Use leaflet at Dermocosmética PTY LTD, Australia.
Made in Italy. VISCODERM® HYDROBOOSTER is a registered trademark of IBSA. IBSA Farmaceutici S.r.l Via Martiri di Cefalonia 2 26900 Lodi – Italy. Distributed in New Zealand by Dermocosmética (NZBN 9429050049066) Suite A, Floor 8 Harbourview Building, 152 Quay St, Auckland Central, Auckland, New Zealand, through Healthcare Logistics. 58 Richard Pearse Drive, Airport Oaks, Manukau City, Auckland, New Zealand.
Copyright © Dermocosmetica 2024. All rights reserved. No part of this publication may be stored in a retrieval system or reproduced by any means whatsoever without written consent of the publisher.
ASPECT DR SKIN PEEL
Whilst amazing results have been observed from chemical skin peel treatments, please know that results vary and we cannot make guarantees. We recommend coming and speaking to our skin specialist for an in-depth consultation. They will discuss in detail how the treatment works, whether it will be right for your skin type and the expected results in line with your skin goals, skin type and skin condition.
PDO THREADS
There are a number of potential risks that you should be aware of prior to being treated with MINT MONO.
Whilst cosmetic treatments such as MINT MONO are effective in most cases, there is a risk that it won’t be effective in your case or that the outcome will not be what you hoped for. The results of this procedure may not fully meet your expectations. Failing to achieve the outcome you hoped for or experiencing side effects may have a psychological impact as well as a physical one.
As with other similar treatments there are a number of possible side effects associated with MINT MONO. These side effects include but are not limited to:
- Bruising: patients should anticipate bruising with this treatment
- Redness for several hours following treatment
- Pain and discomfort: throughout the procedure and patients may experience some discomfort for several days afterwards as the threads settle
- Localised swelling can occur after the treatment, however this should settle in 24 hours after the treatment
- From time to time there are some unexpected complications with this treatment, these include but are not limited to:
- Infection: It is rare for the skin to become infected after a procedure due to its rich blood supply. However, any time you break the skin barrier there is a risk of infection
- Nerve pain/neuralgia: Any time you are inserting an instrument or device beneath the skin (including surgery) there is a risk that you may brush past a nerve causing temporary irritation/aggravation of a nerve. This has a higher risk in certain areas of the face, your practitioner should discuss ‘at risk’ treatment areas with you during your consultation. Hypothetically there is a very remote risk of long-term pain.
- Keloid formation: This is more common in young people. Talk to your practitioner about keloid tendencies.
- Hypersensitivity to PDO – although very rare it is possible for people to develop granulomas as a reaction towards the filament
- If any of the above-mentioned symptoms or signs occur, please alert your treatment provider or clinic immediately.
